Sign up for daily news updates from CleanTechnica on email. Or follow us on Google News!
Carbon capture is back on the hype cycle, years after it was last being pushed upon us as the only real solution to our climate change problem because <insert reason to delay real action here>. It’s a favorite of the fossil fuel industry for obvious reasons. Some of them are even saying the quiet part out loud, that it gives them the social license to not only continue to operate, but to actually increase extraction of coal, oil, and gas.
Meanwhile, the climate crisis is heating up. For the first time, recorded temperatures for the world for the past year averaged 1.5° Celsius more than the pre-industrial reference period. Naturally, more extreme weather, more floods, more fires, more heat waves.
Into this fraught environment filled with rich special interest groups facing the heat deaths of their business models and people who really are trying to do the best thing possible comes more oceanic geoengineering. I’d looked at the concept of geoengineering moderately closely years ago and concluded that if we had to do solar geoengineering, we’d have lost the battle. Creating a higher albedo for the earth would reduce atmospheric temperatures, but it wouldn’t address the root causes and it wouldn’t do anything for the other symptoms of them.
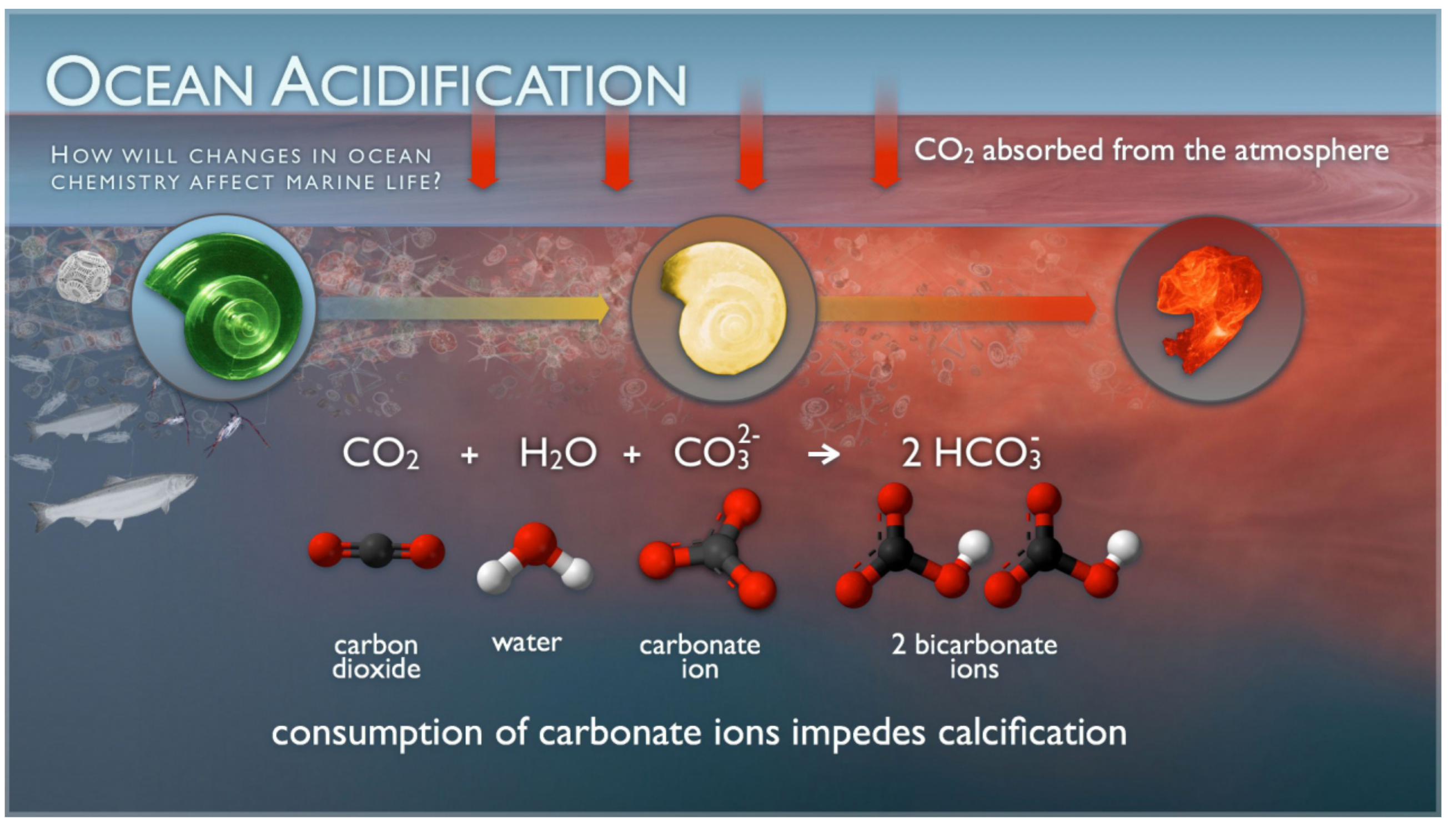
And one of the major symptoms I was concerned about was what is referred to as oceanic acidification. The diagram above illustrates the problem. Carbon dioxide is absorbed the ocean and becomes carbonic acid, hence acidification. But it really just makes the ocean a little less alkaline. That’s actually not directly having any significant effects beyond slightly reducing the ability of the ocean to absorb carbon dioxide, reducing its effectiveness as a carbon sink.
No, it’s what happens with carbonic acid that’s the problem. It grabs carbonate ions and makes bicarbonate ions out of them. One carbon atom from the carbon dioxide grabs another carbon atom from the carbonate ion and makes two bicarbonate ions.
And shellfish need the carbonate ions to build their shells. No carbonate ions, no shells.
I’ve been on a bit of a jag assessing purported ocean carbon drawdown solutions and so far I’m not impressed. First up was Planetary Technologies, which was formed around the idea of dumping milk of magnesia — magnesium hydroxide — in the ocean to bind with the carbonic acid. That actually had merit for the shellfish, but as a carbon drawdown solution, it was dead in the water.
Remember that the slight acidification reduced carbon dioxide update. Magnesium hydroxide bonds with the carbonic acid to make magnesium carbonate and increases alkalinity, enhancing carbon dioxide update ability a bit and not blocking carbonate ions that shellfish need. But magnesium hydroxide costs hundreds of dollars per ton, enhances oceanic uptake only a bit more than a ton of the product, and has a carbon debt of manufacturing that is well above any carbon dioxide uptake benefits. Robbing Peter to pay Paul doesn’t scale unless you found a religion and your name is Paul.
Then I looked at Captura, which reverses the process, breaking apart bicarbonates and carbonate ions electrochemically to create gaseous carbon dioxide. Removing carbon from the big carbon sink in order to create gaseous carbon dioxide that has to be resequestered is a bit of a headscratcher. Especially when you do the math and realize that it would take billions of tons of seawater to get a million tons of carbon dioxide. Just lifting the required seawater for a million tons of carbon dioxide about five meters — from below low tide level to an onshore processing facility — would require about as much electricity as would be required for four million US homes. Just using the electricity to decarbonize the grid would avoid at minimum 17 times the carbon dioxide emissions as might be captured by the Captura solution. The solution likely also has significant membrane problems that they will find very challenging to overcome.
 Chip in a few dollars a month to help support independent cleantech coverage that helps to accelerate the cleantech revolution!
Chip in a few dollars a month to help support independent cleantech coverage that helps to accelerate the cleantech revolution!
And now a contact has pointed me at Equatic, another California solution. There must be something in the water down in the Golden State. All three of the firms have roots there, with Planetary’s CTO being a California professor and Captura and Equatic being founded out of California universities.
What does Equatic do? It takes carbon dioxide from somewhere, so that has to be captured by some other process. Then it sucks seawater out of the sea and mixes the carbon dioxide with it. Then it uses a bit of electricity to mess with the electrochemistry.
Then the carbon dioxide to carbonic acid process that scoops up carbonate and calcium ions along with other ions is accelerated a lot and the water becomes heavy with stable bicarbonate ions. Then this carbonate and calcium ion depleted water is returned to the ocean.
If you’ve been following along, at this point you’ll be asking a question: But what about the shellfish? That’s a really good question which no one seems to be asking the Equatic people. No, they are getting glowing press because their process is going to be piloted in Singapore with a tiny plant that would remove 100 kilograms of carbon dioxide per day and produce a bit of low carbon hydrogen in the process, assuming it was run on green electricity.
Yes, electrolyzing water produces hydrogen. Who knew?
The plant basically is a different hydrogen electrolysis technology that will produce 300 kilograms of hydrogen when a bunch of seawater is mixed with the carbon dioxide. How much seawater? Well, their most recent published paper asserts that they will sequester about 4.6 kilograms of carbon dioxide per cubic meter of of seawater processed. A cubic meter weighs a metric ton, so sequestering a ton of carbon dioxide requires about 217 tons of seawater. That ratio is an order of magnitude better than Captura’s process, so the electricity required just for pumping the water is probably only sufficient to power 400,000 homes for a year and still won’t break even on just using green electricity to green the grid instead.
But you do get green hydrogen out of it, so there’s an upside. Scaling their solution to a million tons of carbon dioxide a year would require 273 times more of everything per day. 100 kilograms of carbon dioxide becomes 27 tons of carbon dioxide per day, 82 tons of hydrogen a day, and 60 tons of sea water a day.
But what about the shellfish?
The same problem Captura has exists with this solution. It depletes the carbonate ions that shellfish require, but it’s more efficient at it. The diffusion problem which would see Captura sucking in carbonate and bicarbonate depleted water for diminishing returns would be amplified here.
So this would require even more careful siting for significant lateral flows of sea water, sideshore currents, in order to avoid diminishing returns and be careful not to be sited anywhere where there were shellfish beds of any significance downstream.
But surely this problem should be front and center for the scientists and advisors of Equatic? Surely they have a biologist on staff? Apparently not.
The most recent paper has a section on the effects on seawater chemistry, but absolutely nothing about the implications of those changes. No article or study I’ve been able to find indicates that they are thinking about this at all.
Of course, I could be wrong. I’m not a marine biologist or an expert in shell formation among mollusks and crustaceans, I’m merely reading highly credible sources and looking at the basic chemistry involved in both the carbon capture solutions and shellfish shell formation and drawing a line between two very closely adjacent dots.
Equatic appears to avoid some of the Captura traps, especially by avoiding membranes and lowering the volume of water required for the process. And their carbon dioxide feed doesn’t need to be perfect, with even 30% carbon dioxide by volume being adequate for the process to perform. And they get valuable hydrogen out, just like the chloralkali process they discuss for comparison, one I looked at related to the only hydrogen for energy play I’ve analyzed that makes sense, Teralta’s diversion of waste hydrogen to avoid burning natural gas at a nearby pulp and paper mill.
The process, if I’m reading the mass energy balances and paper right, reduces the electricity required for electrolysis by changing the alkalinity with carbon dioxide. Basically, they are trading pumping lots of seawater and carbon dioxide for lowered electricity costs, which means that they are very sensitive to the price of carbon dioxide, just as synthetic fuels are.
I’m left with significant red flags. Their process solution will be subject to all the same fouling as anything which runs a lot of seawater through it, so will be higher rather than lower maintenance. Their carbon dioxide stream has to be from a source that doesn’t introduce a lot of other unwanted contaminants, so while it doesn’t have to be pure, it has some significant limitations. It has to be cheap too. And the process accelerates the process of taking shellfish-required chemicals out of seawater.
Have a tip for CleanTechnica? Want to advertise? Want to suggest a guest for our CleanTech Talk podcast? Contact us here.
Latest CleanTechnica TV Video
CleanTechnica uses affiliate links. See our policy here.